Advertisement
Advertisement
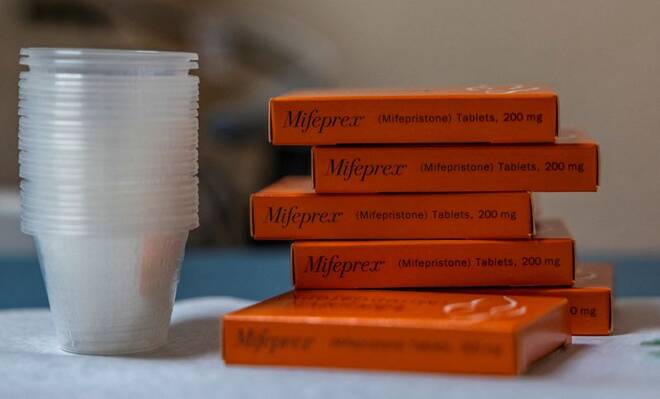
Judge mulls banning abortion pill in US, questions regulatory approval
By:
By Brendan Pierson (Reuters) - A U.S. judge in Texas is set to hear arguments on Wednesday in a bid by anti-abortion groups to ban sales of the abortion pill mifepristone across the country, even in states where abortion is legal, as they challenge regulatory approval granted more than two decades ago.
By Gabriella Borter and Brendan Pierson
AMARILLO, Texas (Reuters) -A U.S. judge on Wednesday questioned lawyers for President Joe Biden’s administration on whether the federal regulatory approval given to the abortion pill mifepristone 22 years ago was proper as he considered a request by anti-abortion groups to ban sales of the drug nationwide.
U.S. District Judge Matthew Kacsmaryk during a hearing in Amarillo also pressed the groups, led by the Texas-based Alliance for Hippocratic Medicine, to explain how he could reverse approval of a long-established drug.
The judge raised the possibility of a more limited ruling, keeping the drug on the market but re-imposing some restrictions lifted by Biden’s administration, including requiring it to be dispensed in person rather than by mail. Kacsmaryk, appointed to the bench by former President Donald Trump, said he would rule “as soon as possible.”
It is shaping up as the most consequential abortion case since the U.S. Supreme Court, powered by its conservative majority, last year overturned its landmark 1973 Roe v. Wade ruling that had recognized a constitutional right to terminate a pregnancy.
The anti-abortion groups sued the U.S. Food and Drug Administration in November, contending the agency used an improper process when it approved mifepristone in 2000 and did not adequately consider the drug’s safety when used by girls under age 18.
The plaintiffs are asking Kacsmaryk for a preliminary order halting sales of mifepristone nationwide – even in states where abortion is legal – while their lawsuit proceeds.
Twelve of the 50 states now ban abortion outright while many others prohibit it after a certain length of pregnancy, according to the Guttmacher Institute, a research organization that supports abortion rights. A ruling against the FDA would hinder abortion access in every state as medication abortion – with mifepristone part of a two-pill regimen – accounts for more than half of U.S. abortions.
The judge heard arguments in a windowless courtroom in a small courthouse in the northwest corner of Texas for more than four hours, listening intently and asking questions.
Erik Baptist, a lawyer with the conservative legal group Alliance Defending Freedom representing the plaintiffs, said the scope of the judge’s ruling should be “universal and nationwide.”
The judge questioned lawyers for Biden’s administration on how the FDA accelerated its approval for mifepristone under a process typically used for drugs to treat HIV infection and other life-threatening illnesses. The administration has said that the drug’s approval was well supported by science, and that the challenge comes much too late.
‘PUBLIC HARM’
Lawyers for the U.S. Justice Department and an attorney for mifepristone’s manufacturer, Danco Laboratories, argued that the plaintiffs had no standing to bring the case, and said mifepristone has an impressive safety and efficacy record.
“An injunction here would upend the status quo. An injunction would cause significant public harm,” Justice Department attorney Julie Straus Harris told the judge.
Harris also argued that a ruling in favor of the plaintiffs would undercut trust in the FDA, the agency charged with signing off on the safety of food products and drugs in the United States. Harris said such a ruling would also increase the burden on surgical abortion clinics, already overcrowded as they admit patients from states where clinics have closed in the wake of last year’s Supreme Court decision.
Mifepristone is available under the brand name Mifeprex and as a generic. Used in conjunction with another drug called misoprostol, it is approved to terminate a pregnancy within the first 10 weeks of a pregnancy. The FDA in January said that the government for the first time will allow mifepristone to be dispensed at retail pharmacies.
Major medical organizations, including the American College of Obstetricians and Gynecologists, have weighed in on the side of the FDA, saying mifepristone “has been thoroughly studied and is conclusively safe.”
Abortion rights supporters, contending that the lawsuit is a baseless attempt to slash abortion access, protested outside the courthouse on Wednesday morning. One dressed as a kangaroo and carried a gavel, suggesting that the hearing was a “kangaroo court.”
By suing in Amarillo, where the Alliance for Hippocratic Medicine had been incorporated just three months earlier, the plaintiffs ensured that the case would go before Kacsmaryk, a conservative former Christian activist. His courthouse has become a favored destination for Republicans seeking to challenge aspects of Democrat Biden’s agenda.
Kacsmaryk’s eventual ruling is likely to be appealed immediately by the losing side to the New Orleans-based 5th U.S. Circuit Court of Appeals, with the U.S. Supreme Court a possible next step after that.
The 5th Circuit has a conservative reputation, with more than two-thirds of its judges appointed by Republican presidents. The Supreme Court has a 6-3 conservative majority.
(Reporting Gabriella Borter in Amarillo, Texas and Brendan Pierson in New York; Additional reporting by Liliana Salgado in Amarillo; Editing by Will Dunham and Alexia Garamfalvi)
About the Author
Reuterscontributor
Reuters, the news and media division of Thomson Reuters, is the world’s largest international multimedia news provider reaching more than one billion people every day. Reuters provides trusted business, financial, national, and international news to professionals via Thomson Reuters desktops, the world's media organizations, and directly to consumers at Reuters.com and via Reuters TV. Learn more about Thomson Reuters products:
Advertisement